
The enthalpy difference between α- and α-graphite is less than 1 kJ mol -1 (0.59 ± 0.17 kJ mol -1. although the carbon-carbon distances and the interlayer spacing remains the same as in the α-form. repeat fashion but the β-form (rhombohedral) the stacking is ABCABCABC. In most graphite (α-graphite), the layers of atoms are arranged in an ABABAB. The distance between the layers of carbon atoms is 335.4 pm. Delocalization in the bonding is evident since the C-C distances are equal and shorter than normal carbon-carbon single bonds (typcally 154 pm).
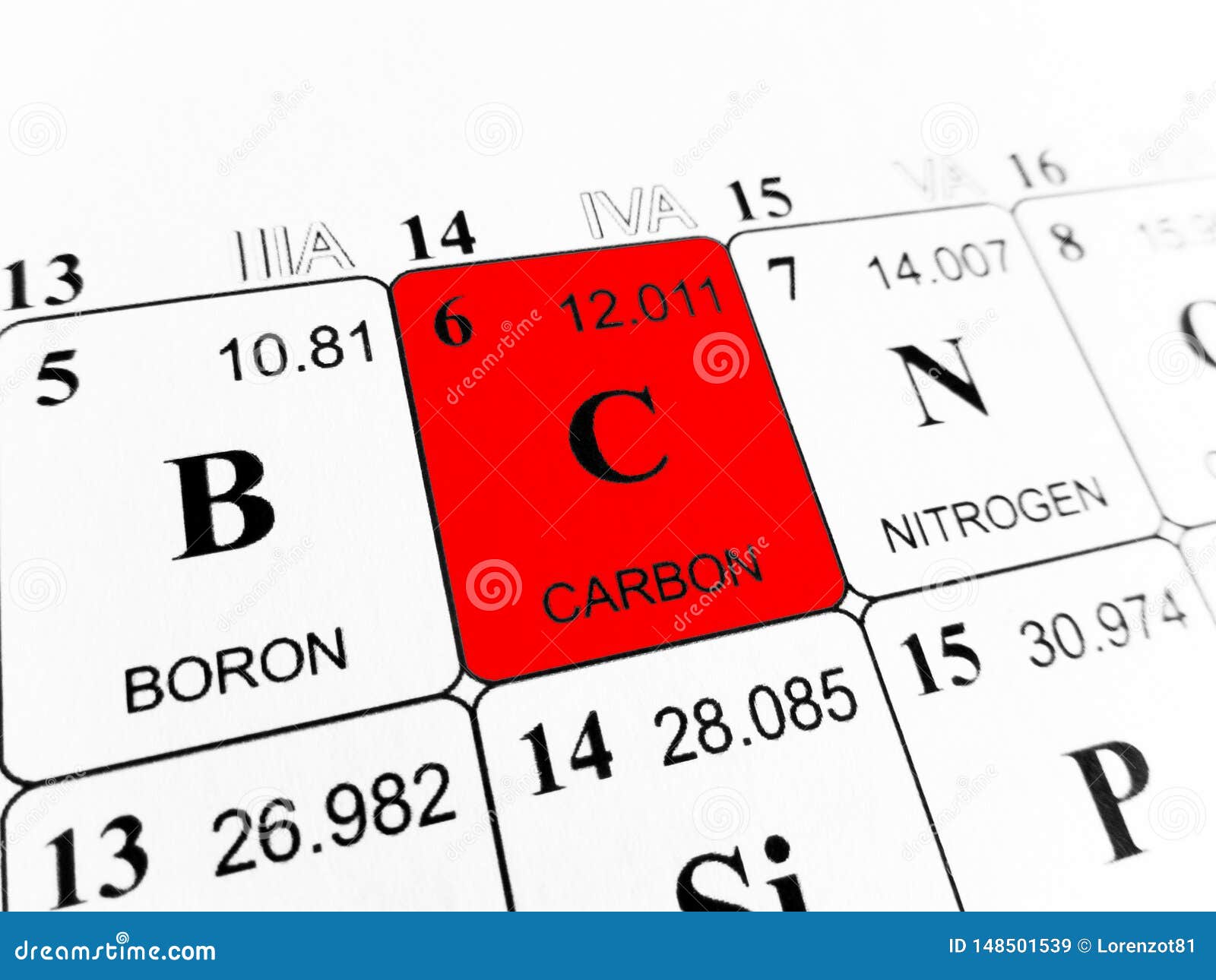
Most graphite is α-graphite and it possesses a layer structure in which each carbon is directly bound to three other carbon atoms at a distance of 141.5 pm. Amorphous forms of carbon such as soot and lampblack are materials consisting of very small particles of graphite. Whereas diamond and graphite are infinite lattices, fullerenes such as buckminsterfullerene, C 60, is a discrete molecular species. Other forms of carbon include the fullerenes. Diamond is a second form of carbon but is much less common. This is also the thermodynamically most stable form. The most common form of pure carbon is α-graphite. Pure carbon is available in a number of different forms (allotropes). This form of carbon is the subject of great interest in research laboratories today. More recently, another form of carbon, buckminsterfullerene, C 60, was discovered. Diamonds are also recovered from the ocean floor off the Cape of Good Hope. Natural diamonds are found in ancient volcanic "pipes" such as found in South Africa. Carbon, as microscopic diamonds, is found in some meteorites. Graphite is one of the softest known materials while diamond is one of the hardest.
#CARBON PERIODIC TABLE KEY FREE#
While silicon might take the place of carbon in forming a host of related compounds, it is not possible currently to form stable compounds with very long chains of silicon atoms.Ĭarbon is found free in nature in three allotropic forms: amorphous, graphite, and diamond. Organic chemistry, a 1/112th subset of inorganic chemistry, is the study of carbon and its compounds. Carbon is unique among the elements in the vast number of variety of compounds it can form. The atmosphere of Mars is very thin but what there is contains about 95 % CO 2.Ĭoal, petroleum, and natural gas are chiefly hydrocarbons. It is a component of rocks as carbonates of calcium (limestone), magnesium, and iron.
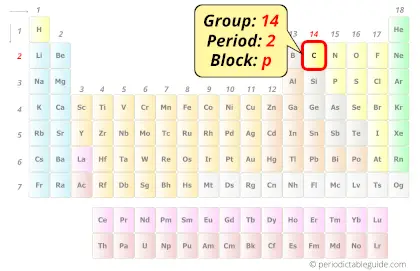
Carbon is present as carbon dioxide in the atmosphere and dissolved in all natural waters. It is found in abundance in the sun, stars, comets, and atmospheres of most planets. Carbon is a Group 14 element and is distributed very widely in nature.


 0 kommentar(er)
0 kommentar(er)
